Introduction
These concentrate formulas are developed by Alex Levitt as an easier to use and easier to experiment with way of remineralizing distilled or reverse osmosis water for brewing coffee. They feature generally sane numbers that you can work out with only mental math and verify with cheap and easily obtainable tools.
Why Use These?
Existing Options
BaristaHustle introduces an extremely helpful guide to mixing your own water in the post The World in Two Bottles, although I feel like there are a couple ways the method could be better:
- The recipes use ppm expressed as ion rather than ppm expressed as CaCo3, which makes it more difficult to check your results with a TDS meter.
- The recipes require weird weights while mixing the concentrates into your final brewing water.
- The recipes require weighing out water as well as concentrates.
There are also premixed options, such as Third Wave Water (TWW) and Global Customized Water (GC Water). But these options are more expensive than mixing your own, and have less control if you wish to make any tweaks.
What this method adds
Alex’s recipes raise the ppm (expressed as CaCo3) of your brewing water by 10ppm for every 1ml of concentrate added. Thus allowing for both easier intuitive mixing, and the ability to easily check your numbers with a TDS meter. Want 40ppm calcium hardness and 20ppm alkalinity? Just add 4ml and 2ml of your calcium and alkalinity concentrates respectively, then check that it reads 60ppm. Checking after each addition (confirming your 4ml raises the ppm to 40 and the 2ml increases it to 60) also lets you verify your concentrates are as they should be.
There are concentrates for both 1L of brewing water and 1gal of brewing water, depending on your preference. With a little math, you can tweak them to other sizes too. Whether you want to weigh out your water, or dump the concentrates right in a gallon straight off the shelf you can.
What You’ll Need
At Least
- One hardness source. Magnesium sulfate is a good start.
- One alkalinity source. Sodium bicarbonate is a good start.
- Distilled water for concentrates and brew water.
- Something to store your concentrates in. Mason jars, Pyrex flasks, whatever. Make sure whatever you’re using has inert (plastic, silicone, etc) lids. Metal lids can corrode because of the super concentrated minerals in the water.
- Something to store your brew water in. Can be a liter or a gallon, the container it came in, or a dedicated container.
- A scale accurate to at least .1g for dry ingredients and liquids.
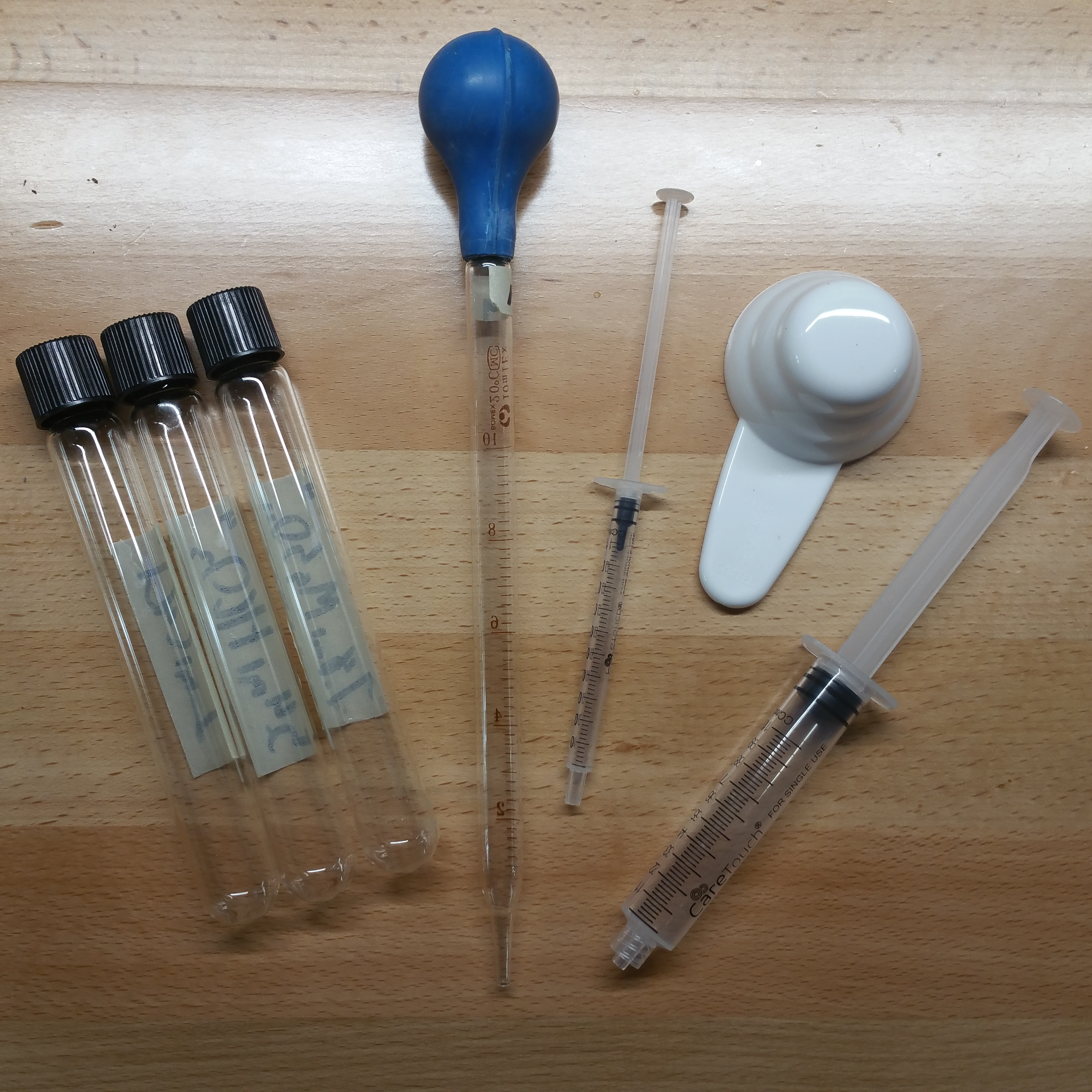
- A way to move liquid from your concentrates to your brew water: pipettes, syringes, etc.
- A TDS meter to check your results, I like my COM-100, but even a cheap pen one will let you know you’re about where you should be.
Optionally
- More hardness sources such as calcium chloride or magnesium chloride.
- More alkalinity sources such as potassium bicarbonate.
- A scale accurate to .01g or .001g for better accuracy. I use this one. An Acaia Lunar can also be set to .01g in a hidden menu setting if you have one of those.
Making Concentrates


Note the plastic lids as mentioned above.
In addition, Alex’s concentrates are modified such that 1ml adds 5ppm instead of 10ppm.
Mixing Concentrates
Simply weigh out the dry ingredients as instructed, place them in the concentrate bottle, and add the correct amount of distilled water. Water is given as “this amount of water in addition to the dry weight”, not “total weight including dry components and water”. Mix until fully dissolved, and ideally let rest a few hours before using to ensure everything is fully dissolved. You shouldn’t see any crystals in the water, though certain minerals like CaCl2 may be appear cloudy. If you want to speed that up, you can use hot water. Somewhere in the “hot enough it’s uncomfortable to touch but not so hot it’s dangerous to touch” range for increasing speed without also causing too much evaporation.
You can customize further if you like too, with a little math. But I generally recommend starting off as simple as possible. When you’re ready to try some more, check out the collapsed section below.
Modifying Concentrates (Optional)
Basic Changes
If you want to make larger or smaller concentrates than these 800ml ones, you can simply multiply or divide both the dry component and the water by the same number. (Half the liter recipes for .5L, double them for 2L, etc.)
The same also works to change the amount of hardness you want to add if, for example, you’d prefer concentrates that add 5ppm per 1ml instead of 10ppm.
You can, with the same method, also scale the concentration down so that 1ml adds 10ppm to any size body of water, be it a .5L kettle or a 150ml cup. You can scale up as well for bodies of water larger than 1 gallon, but you’re generally more limited than going down, since at some point the minerals will no longer fully dissolve. You could maybe push it to 4L, but not much further.
But I only recommend doing so if you primarily mineralize that same volume (e.g. you always add concentrates directly to your 500ml kettle), since there’s an easier way for occasional differences in volume.
Dilution
That method is dilution of the concentrates to mineralize a different volume of water. This allows you do perform experiments involving mixing water for just one kettle or even just one cupping bowl without having to get your powders out again. It is slightly more math, but don’t worry, there’s nothing complicated. For my example here, we’ll be assuming a gallon concentrate, since I imagine it will be the most frequently used.
We need a dilution factor, from which we’ll subtract 1ml to find the amount of distilled water to dilute with, found with the equation:
\[
\boxed{\frac{3785ml}{Xml}=Dilution\; Factor}
\]
If you’re diluting concentrates for a 500ml kettle, your equation will look like this:
\[
\boxed{\frac{3785ml}{500ml}=~7.5}
\]
This means to make concentrates, you’ll add 6.5ml of distilled water for every 1ml of gallon concentrate. For example, you could mix 65ml of distilled water with 10ml of gallon concentrate to make up 75ml of concentrate for a 500ml kettle. Each 1ml of this 75ml concentrate will raise the hardness of a 500ml kettle of water by 10ppm.
If you’re diluting concentrates for a 150ml cupping bowl, your equation would look like:
\[
\boxed{\frac{3785ml}{150ml}=~25}
\]
So for every 24ml of distilled water, we add 1ml of gallon concentrate. Just like the other concentrates, 1ml will raise the hardness of the intended volume by 10ppm.
Hardness Sources
These minerals increase the general hardness of the water. Higher hardness is generally thought to increase sweetness and extraction, but also bring out more defects from the coffee too should any be present. Although there is some debate around this, and I’d like to run a serious experiment before making a definite claim.
You’ll often see people refer to this as general hardness, often abbreviated to GH. I won’t be using that terminology here. But if you see GH used in the context of coffee, it’s safe to assume we mean the same thing.

Magnesium Sulfate Heptahydrate
MgSO4 x 7H2O
74.6g in 800ml distilled water for a 1 gallon concentrate.
19.7g in 800ml distilled water for a 1 liter concentrate.
Sold as Epsom salt. Or you can order some HERE.

Calcium Chloride Anhydrate
CaCl2
33.6g in 800ml distilled water for a 1 gallon concentrate.
8.9g in 800ml distilled water for a 1 liter concentrate.
You can order some HERE.

Magnesium Chloride Hexahydrate
MgCl2 x 6H2O
61.6g in 800ml distilled water for a 1 gallon concentrate.
16.3g in 800ml distilled water for a 1 liter concentrate.
You can order some HERE.
Alkalinity Sources
These minerals increase the alkalinity of the water, also known as buffer. Alkalinity is generally thought to protect coffee from unpleasant acidity.
Sometimes you’ll see people refer to alkalinity as carbonate hardness, often abbreviated to KH. I won’t be using that terminology here, and if you’re interested in why, again check out the section below.
Why do we use Alkalinity instead of KH?
In the context of coffee, KH or carbonate hardness will usually just mean buffer, but they are not actually the same thing.
The term “alkalinity” has to do with the ability of a water to buffer acid. The term KH has to do with the potential of a water when boiled to form scale.
They can be equal, and often will be in brewing water, but don’t necessarily have to be.

Sodium Bicarbonate
NaHCO3
50.9g in 800ml distilled water for a 1 gallon concentrate.
13.4g in 800ml distilled water for a 1 liter concentrate.
Sold as baking soda. Or you can order some HERE.

Potassium Bicarbonate
KHCO3
60.6g in 800ml distilled water for a 1 gallon concentrate.
16.0g in 800ml distilled water for a 1 liter concentrate.
You can order some HERE.
Mixing Brew Water
How is our water described
- Hardness: The total ppm added by the minerals in the hardness category.
- Alkalinity: The total ppm added by the minerals in the alkalinity category.
- H:A*: The ratio of hardness to alkalinity.
- Total PPM: Your combined hardness and alkalinity (plus anything else that may be in the water if using RO water instead of distilled).
*H, A, and H:A are just me using the first letter of the word and not standards. If you know of a standard abbreviation please let me know.
There can be other things in water, but since we’re mixing our own instead of using tap water, bottled water, or water out of some murky pond, we don’t have to worry about them.
What makes ideal water?
I’m not going to go too into what makes for ideal brew water here or what effects each water recipe has on taste just yet. I’m taking part in some ongoing water blind triangulation experiments right now, and may post recommendations after that. For now, I just want to give you the tools to mix water yourself, and a few recipes as starting points. My best recommendation for now is to try a few, blind cup them, mix some of your own, blind cup them, and look for something you like.
What NOT to do
That being said, be careful not to mix water that’s harmful to your equipment. Chlorides can be corrosive to even stainless steel in too high of quantities, so don’t go crazy with CaCl2 or MgCl2, below 30ppm or so, and probably avoid them altogether if you’re using a brass or copper boiler. Don’t go too hard or you may find something resembling scale, even with nonscaling minerals you may get a powdery coating. And don’t go too pure since that can be damaging to equipment as well. Especially in espresso machines with fill sensors, which distilled water won’t trigger. Experimenting with extremes is great, but I wouldn’t go putting 1ppm or 500ppm in an espresso machine. Honestly, about anything is going to be fine in a kettle, just make sure to empty it and wash it afterward if you use extremely high chlorides.
Equipment For Easier Mixing
You have a few options for moving concentrates to your brew water. I generally like to use 1ml syringes, as they have numbered markings of .1ml and smaller notches in between. Easy to measure exactly how much you’re sucking into the syringe. But you have other options like 10ml syringes or pipettes of various sizes if you want something faster to use. Whatever you use, make sure to flush it with distilled or RO water after every use so that you don’t have minerals drying in it and ruining future batches.
If you’re also weighing water for greater precision than syringes provide, the Hario spoons that come with v60 brewers are extremely hydrophobic and make for excellent weighing vessels. (Shoutout to Jonathan Gagné for the tip!) It’s bizarre how well these things repel water.
Mixing Water
Take concentrates and add to your distilled/ro water 1ml concentrate for every 10ppm hardness you’d like to add of said concentrate, or scale down to .1ml for 1ppm. When you’re done, shake it up and let it sit a little. Check your ppm with the TDS meter, and if it’s about where it should be (the pen ones have a bit of error, but a COM-100 should be pretty spot on) you’re good to go!
That’s it.
Storing Water
I personally leave my concentrates and brewing water out in sealed glass containers and haven’t noticed any ill effects. Others prefer to keep their water in the refrigerator to avoid stagnation. Find what produces the best results for you.
Recipes
Credits
Many, in fact most, of the waters below are not my own original recipes. The recipes are simply translations to get the same end end result, but using the methodology outlined in this guide. If I have any of the conversions wrong, or you would like to submit a water recipe (or have yours taken down) please let me know and I’ll get to it right away!
The water marked with a 1 is a mix of Matt Perger and Scott Rao’s water from Jonathan Gagné’s post, Water for Coffee Extraction, adapted to use this method of mixing.
The water marked with a 2 is a general guideline on mixing water for espresso machines by HomeBarista user Professor Robert Pavlis.
Waters marked with a 3 are from the BaristaHustle post The World in Two Bottles, adapted to use this method of mixing.
Examples
| Water | Total PPM | H:A | MgSO4 | MgCl2 | CaCl2 | NaHCO3 | KHCO3 |
|---|---|---|---|---|---|---|---|
| My Water | 96ppm | 2:1 | 4.4ml (44ppm) | - | 2ml (20ppm) | 3.2ml (32ppm) | - |
| Alex's Water | 80ppm | 3:1 | 2ml (20ppm) | 2ml (20ppm) | 2ml (20ppm) | 2ml (20ppm) | - |
| Rao/Perger Water \(^1\) | 127ppm | 2.2:1 | 4ml (40ppm) | 2ml (20ppm) | 2.7ml (27ppm) | 2ml (20ppm) | 2ml (20ppm) |
| R. Pavlis Water\(^2\) | 30-60ppm | - | - | - | - | - | 3-6ml (30-60ppm) |
| BH Melbourne\(^3\) | 35ppm | 2:1 | 2.3ml (23ppm) | - | - | 1.2ml (12ppm) | - |
| BH SCA\(^3\) | 107ppm | 1.7:1 | 6.7ml (67ppm) | - | - | 4ml (40ppm) | - |
| BH Perger Water\(^3\) | 120ppm | 2:1 | 8mlml (80ppm) | - | - | 4ml (40ppm) | - |
| BH Rao\(^3\) | 124ppm | 1.5:1 | 7.4ml (74ppm) | - | - | 5ml (50ppm) | - |
| BH Hendon\(^3\) | 129ppm | 3.2:1 | 9.8ml (98ppm) | - | - | 3.1ml (31ppm) | - |
| BH Pretty Hard\(^3\) | 159ppm | 3.5:1 | 12.4ml (124ppm) | - | - | 3.5ml (35ppm) | - |
| BH Hard.AF\(^3\) | 219ppm | 3.8:1 | 17.4ml (174ppm) | - | - | 4.5ml (45ppm) | - |